| CAS Registry Number: 106-51-4 Molecular Formula: C6H4O2 |
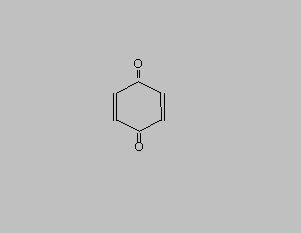 |
QUINONE
Quinone is a federal hazardous air pollutant and was identified
as a
toxic air contaminant in April 1993 under AB 2728.
| CAS Registry Number: 106-51-4 Molecular Formula: C6H4O2 |
 |
Quinone is formed as yellow crystals and has a characteristic irritating
odor like that of chlorine (Merck, 1989). It is slightly soluble in water,
alcohol, ether, hot petroleum ether, and alkalis. Quinone is an oxidizing
agent and is reduced to hydroquinone (HSDB, 1991).
Physical Properties of Quinone
| Molecular Weight: | 108.10 |
| Melting Point: | 115.7 oC |
| Vapor Pressure: | 0.1 mm Hg at 25 oC |
| Vapor Density: | 3.7 (air = 1) |
| Density/Specific Gravity: | 1.318 at 20/4 oC (water = 1) |
| Log/Octanol Water Partition Coefficient: | 0.20 |
| Conversion Factor: | 1 ppm = 4.42 mg/m3 |
B. Emissions
No emissions of quinone from stationary sources in California were reported,
based on data obtained from the Air Toxics "Hot Spots" Program
(AB 2588) (ARB, 1997b).
C. Natural Occurrence
Quinone occurs naturally in a variety of arthropods and many insects synthesize
simple benzoquinones (HSDB, 1991).
AMBIENT CONCENTRATIONS
No Air Resources Board data exist for ambient measurements of quinone.
INDOOR SOURCES AND CONCENTRATIONS
No information on indoor sources and concentrations of quinone was found
in the readily-available literature.
ATMOSPHERIC PERSISTENCE
Quinone will exist in the atmosphere in the gas phase. The dominant atmospheric
loss process for quinone is expected to be by reaction with the hydroxyl
(OH) radical [reaction with ozone is expected to be slow because of the
>C(O) substituent groups (Atkinson and Carter, 1984)]. The estimated
half-life and lifetime of quinone in the atmosphere due to reaction with
the OH radical are about 3 hours and 4 hours, respectively (Kwok and Atkinson,
1995).
AB 2588 RISK ASSESSMENT INFORMATION
Quinone emissions are not reported from stationary sources in California
under the AB 2588 program. It is also not listed in the California Air
Pollution Control Officers Association Air Toxics "Hot Spots"
Program Revised 1992 Risk Assessment Guidelines as having health values
(cancer or non-cancer) for use in risk assessments (CAPCOA, 1993).
HEALTH EFFECTS
Probable routes of human exposure to quinone are inhalation and dermal
contact (HSDB, 1991).
Non-Cancer: Quinone is a severe irritant of the eyes and respiratory tract.
Exposure induces methemoglobinemia. Skin contact can cause skin ulceration
and pigmentation changes (U.S. EPA, 1994a). Acute overexposure may also
cause persons with a history of congenital or acquired eye defects to be
at increased risk from exposure. Persons with poor visual acuity from high
degrees of astigmatism, keratoconus, or existing corneal injury should
be protected from repeated, uncontrolled exposure to quinone vapor (HSDB,
1991). Acute dermal exposure may result in dermatitis.
The United States Environmental Protection Agency (U.S. EPA) has determined
that there are inadequate data to establish a Reference Concentration (RfC)
for quinone, and has not set an oral Reference Dose (RfD) (U.S. EPA, 1994a).
No information is available on adverse reproductive or developmental effects
of quinone in humans or animals (U.S. EPA, 1994a).
Cancer: No information is available regarding the carcinogenicity of quinone
in humans. The U.S. EPA has not classified quinone for carcinogenicity
(U.S. EPA, 1994a). The International Agency for Research on Cancer has
classified quinone (para-quinone) in Group 3: Not classifiable as a carcinogen
(IARC, 1987a).